Biodiesel Production
Key Reaction. The main reaction for converting oil to biodiesel is called transesterification. The transesterification process reacts an alcohol (like methanol) with the triglyceride oils contained in vegetable oils, animal fats, or recycled greases, forming fatty acid alkyl esters (biodiesel) and glycerin. The reaction requires heat and a strong base catalyst, such as sodium hydroxide or potassium hydroxide. The simplified transesterification reaction is shown below.
Triglycerides + Free Fatty Acids (<4%) + Alcohol ——> Alkyl esters + glycerin
Pretreatment Reaction. Some feedstocks must be pretreated before they can go through the transesterification process. Feedstocks with less than 4% free fatty acids, which include vegetable oils and some food-grade animal fats, do not require pretreatment. Feedstocks with more than 4% free fatty acids, which include inedible animal fats and recycled greases, must be pretreated in an acid esterification process. In this step, the feedstock is reacted with an alcohol (like methanol) in the presence of a strong acid catalyst (sulfuric acid), converting the free fatty acids into biodiesel. The remaining triglycerides are converted to biodiesel in the transesterification reaction.
Triglycerides + Free Fatty Acids (>4%) + Alcohol ——> Alkyl esters + triglycerides
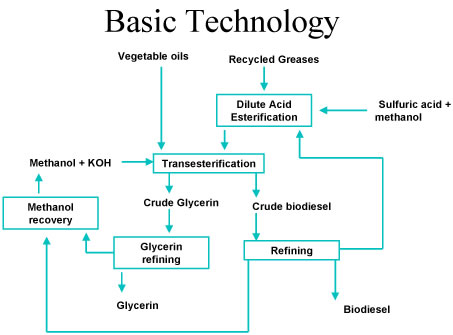
- Acid Esterification. Oil feedstocks containing more than 4% free fatty acids go through an acid esterification process to increase the yield of biodiesel. These feedstocks are filtered and preprocessed to remove water and contaminants, and then fed to the acid esterification process. The catalyst, sulfuric acid, is dissolved in methanol and then mixed with the pretreated oil. The mixture is heated and stirred, and the free fatty acids are converted to biodiesel. Once the reaction is complete, it is dewatered and then fed to the transesterification process.
- Transesterification. Oil feedstocks containing less than 4% free fatty acids are filtered and preprocessed to remove water and contaminants and then fed directly to the transesterification process along with any products of the acid esterification process. The catalyst, potassium hydroxide, is dissolved in methanol and then mixed with and the pretreated oil. If an acid esterification process is used, then extra base catalyst must be added to neutralize the acid added in that step. Once the reaction is complete, the major co-products, biodiesel and glycerin, are separated into two layers.
- Methanol recovery. The methanol is typically removed after the biodiesel and glycerin have been separated, to prevent the reaction from reversing itself. The methanol is cleaned and recycled back to the beginning of the process.
- Biodiesel refining. Once separated from the glycerin, the biodiesel goes through a clean-up or purification process to remove excess alcohol, residual catalyst and soaps. This consists of one or more washings with clean water. It is then dried and sent to storage. Sometimes the biodiesel goes through an additional distillation step to produce a colorless, odorless, zero-sulfur biodiesel.
- Glycerin refining. The glycerin by-product contains unreacted catalyst and soaps that are neutralized with an acid. Water and alcohol are removed to produce 50%-80% crude glycerin. The remaining contaminants include unreacted fats and oils. In large biodiesel plants, the glycerin can be further purified, to 99% or higher purity, for sale to the pharmaceutical and cosmetic industries.

